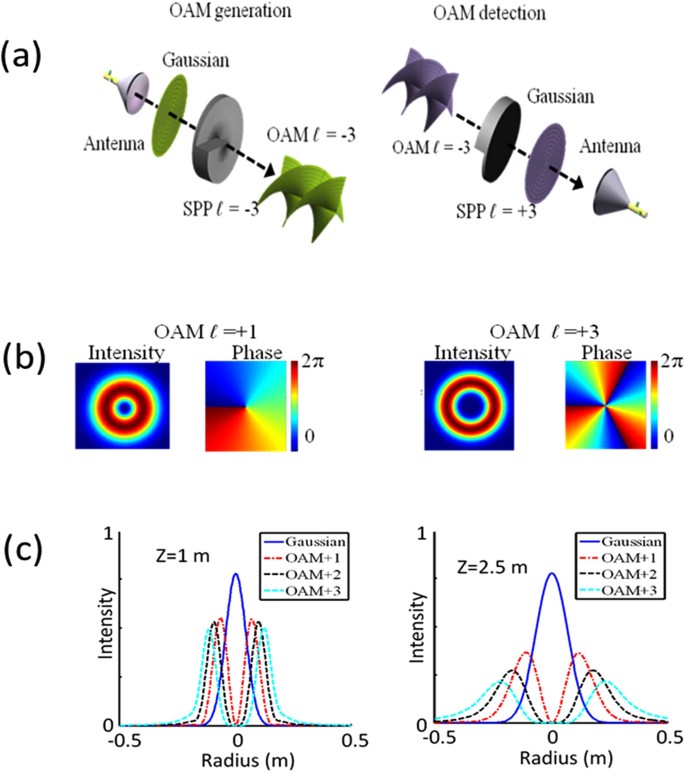
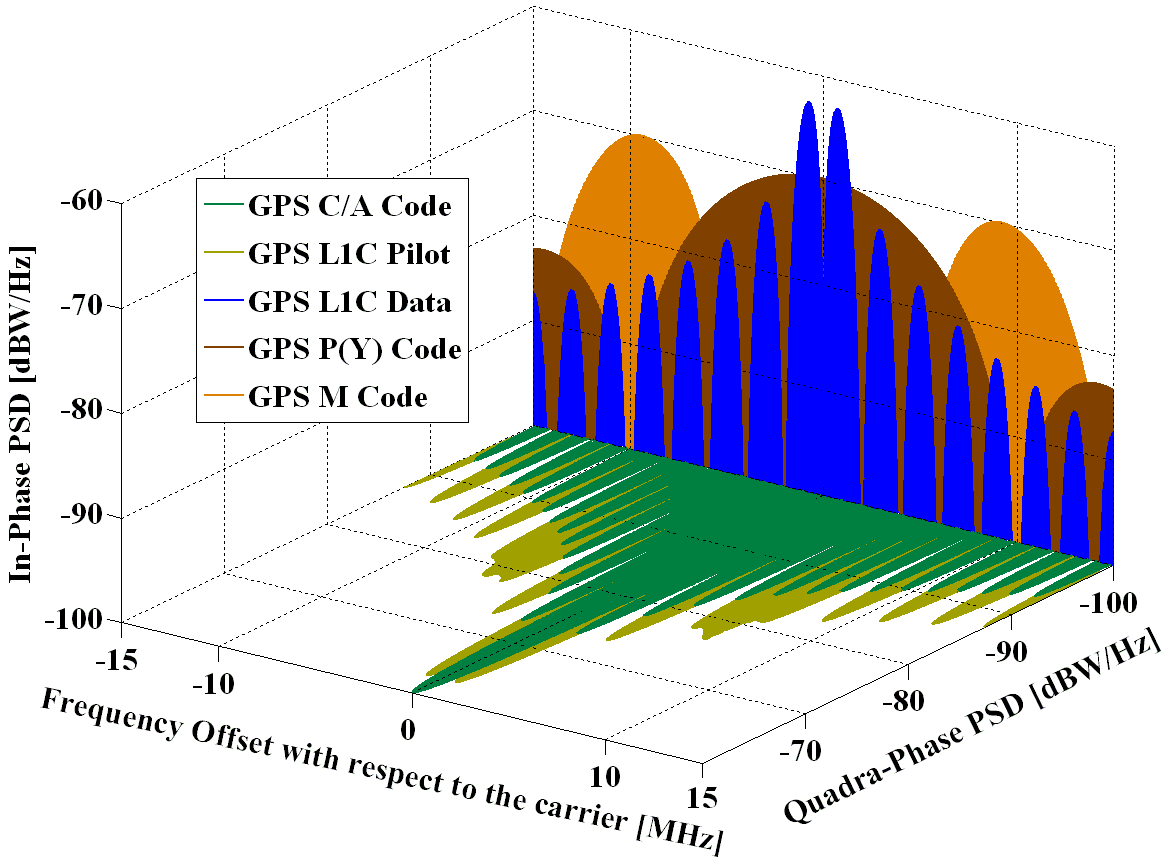
A pendulum of length ℓ = 1 m is released from theta 0 = 60 ^ ∘ . The rate of change of speed of the bob at theta = 30 ^ ∘is ( g = 10 m / s ^ 2 )
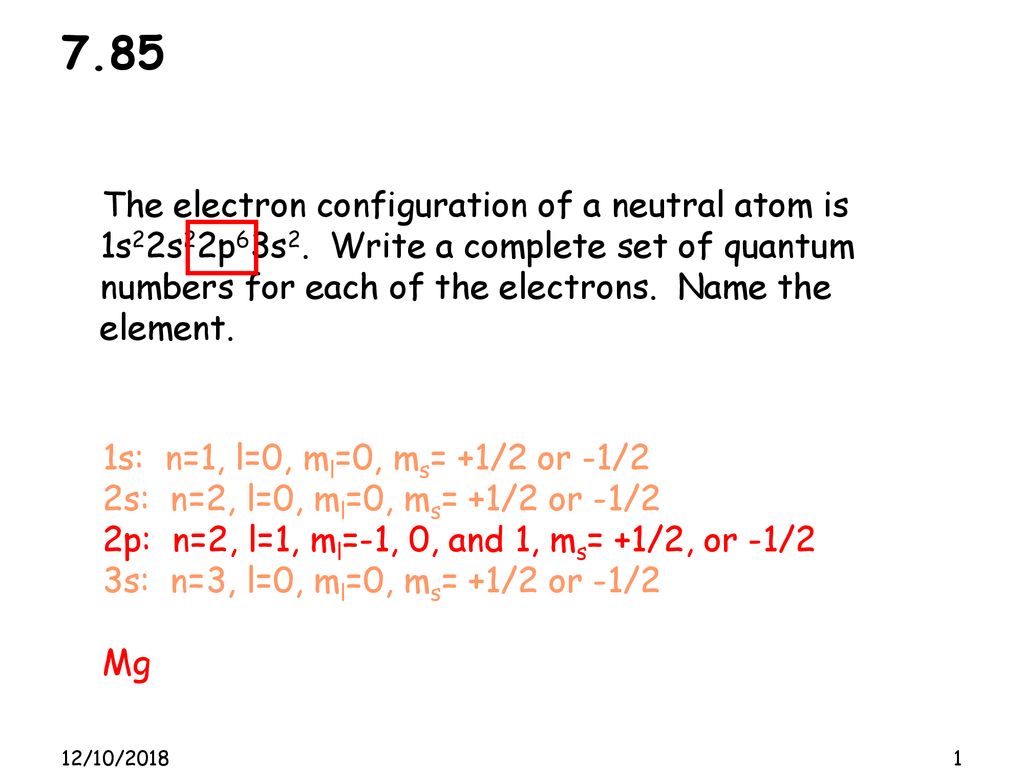
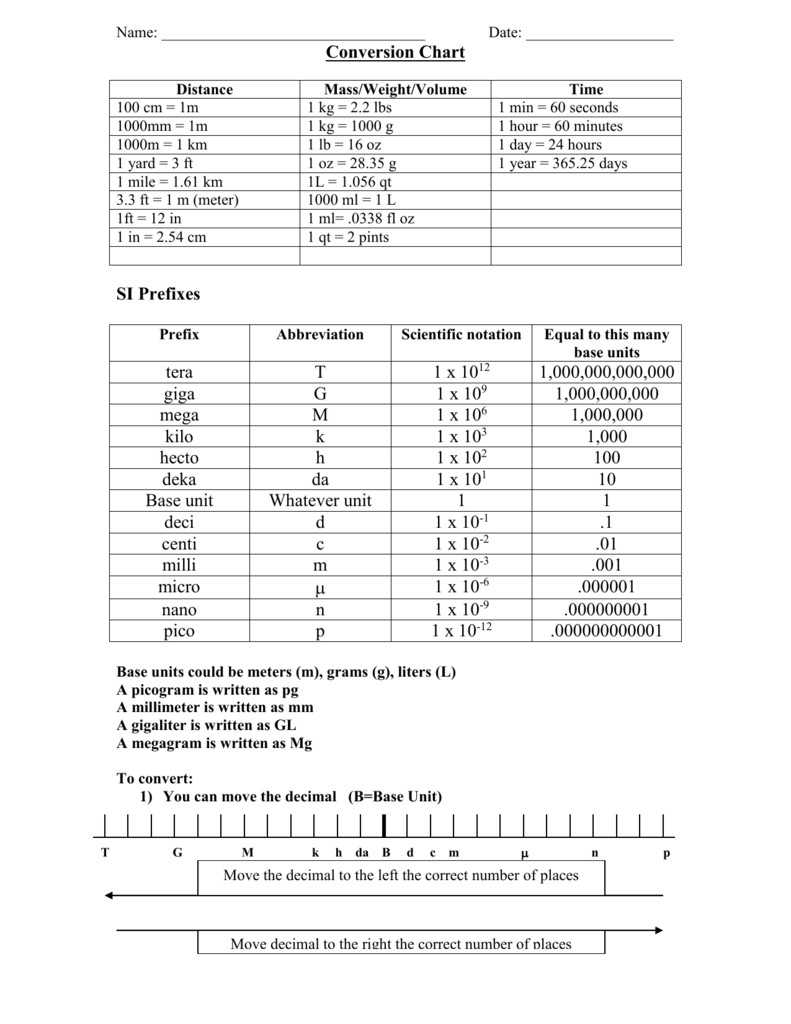
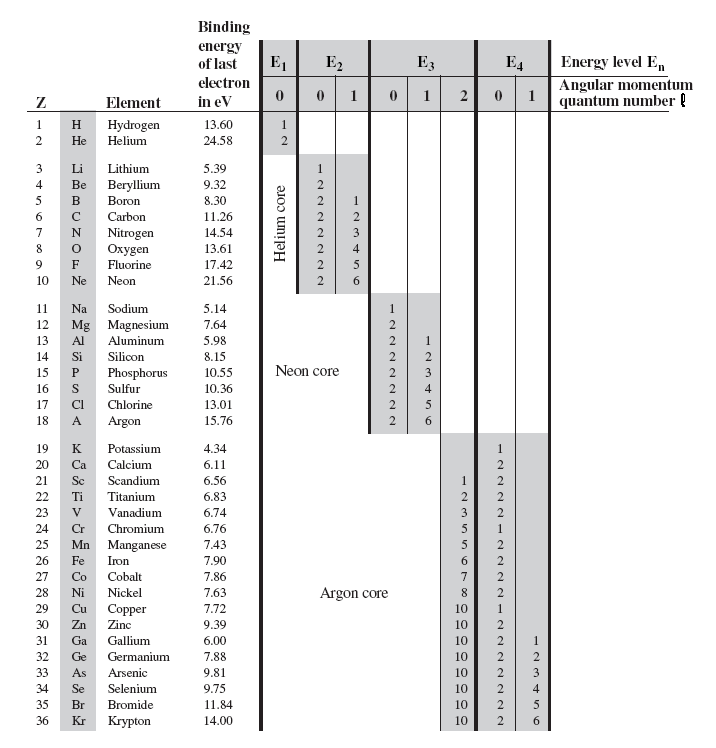
7.85 The electron configuration of a neutral atom is 1s22s22p63s2. Write a complete set of quantum numbers for each of the electrons. Name the element. - ppt download

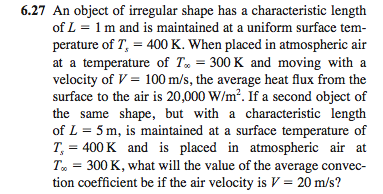
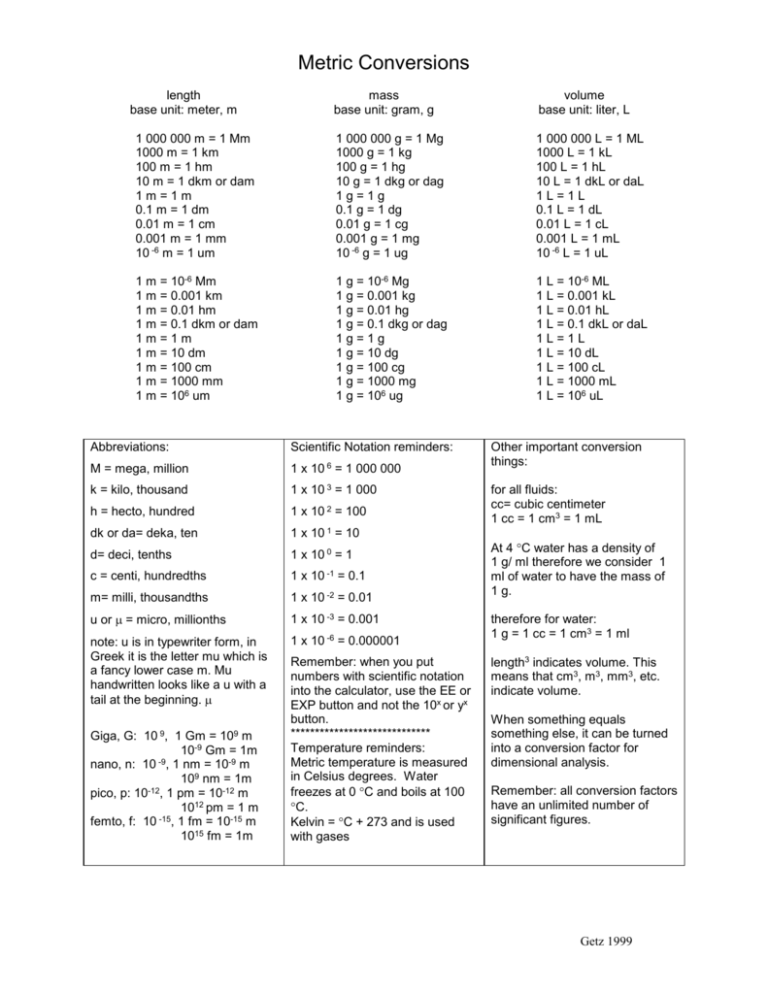
ACID-BASE CHEMISTRY. CONCENTRATION UNITS - I Mass concentrations Water analyses are most commonly expressed in terms of the mass contained in a liter. - ppt download

A pendulum of length ℓ = 1 m is released from theta 0 = 60 ^ ∘ . The rate of change of speed of the bob at theta = 30 ^ ∘is ( g = 10 m / s ^ 2 )
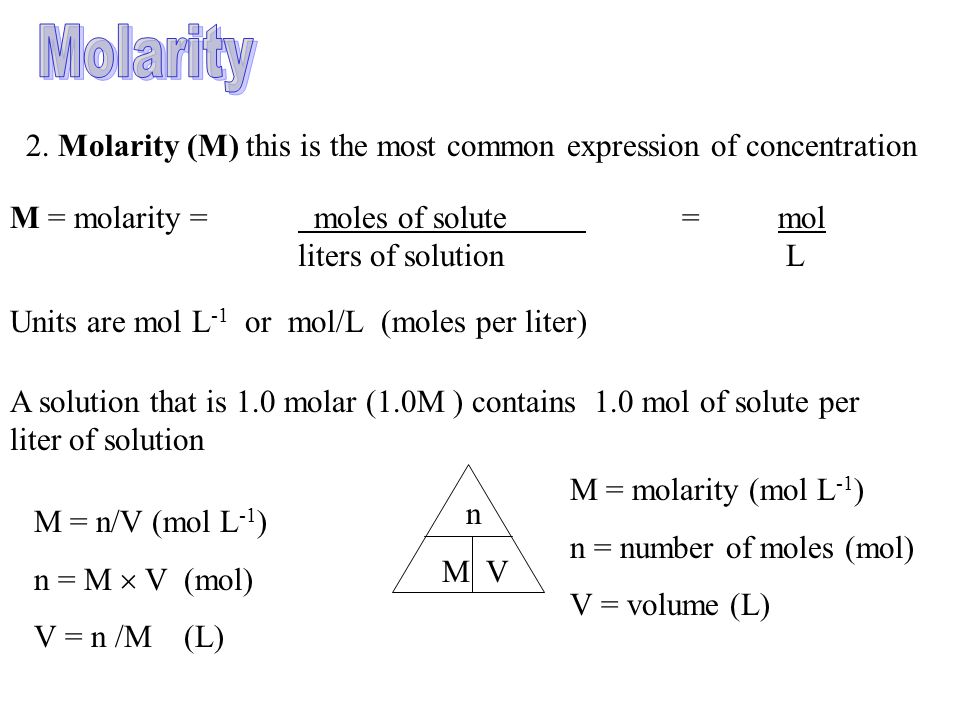
Molarity 2. Molarity (M) this is the most common expression of concentration M = molarity = moles of solute = mol liters of solution L Units are. - ppt download

What is the maximum number of orbitals that can be identified with the following quantum numbers ? n = 3, l = 1, ml = 0.
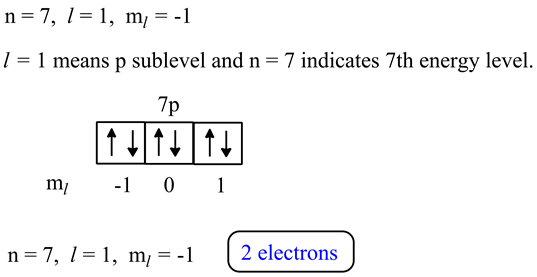
How many electrons in an atom could have these sets of quantum numbers?n=2 --> number electrons =? - Home Work Help - Learn CBSE Forum